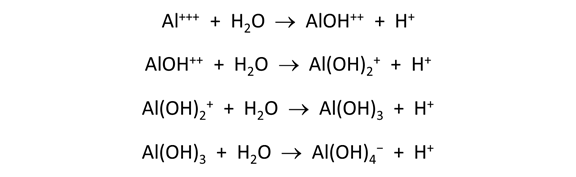Stability diagrams
Stability Diagrams is part of a free web series, GWB Online Academy, by Aqueous Solutions LLC.
What you need:
- GWB Essentials recommended
Download this unit to use in your courses:
- Lesson plan (.pdf)
- PowerPoint slides (.pptx)
Introduction
Act2 and Tact calculate stability diagrams on activity, fugacity, and temperature axes. They additionally calculate solubility diagrams and can be overlain with scatter data from GSS spreadsheets or traces of React runs.
The basis is the set of aqueous species, minerals, and gases with which we choose to
- Write chemical reactions
- Express composition
The number of basis entries is the number of components in the system, which is fixed by the laws of thermodynamics.
By convention, we will choose as the basis
- Water, the solvent
- Each mineral in equilibrium with the system
- Each gas at known fugacity or partial pressure
- Important aqueous species
In any GWB model, you follow a similar set of steps:
- SWAP. Prepare your calculation by swapping into the basis the species with which you wish to write reactions, including any coexisting minerals and gases. For an activity-activity diagram, the basis includes the main species and axis variables.
- SET. Set constraints on the system, perhaps including temperature, total concentration, species' activity, gas fugacity or partial pressure, etc.
- GO. Select Run → Go to let the program trace the reaction path, calculate and display the diagram, or balance the reaction.
- REVISE. Change the basis, add new components, or alter any of the specifications for your model. Then select Run → Go to render the new result.
Task 1 - Activity-activity diagrams
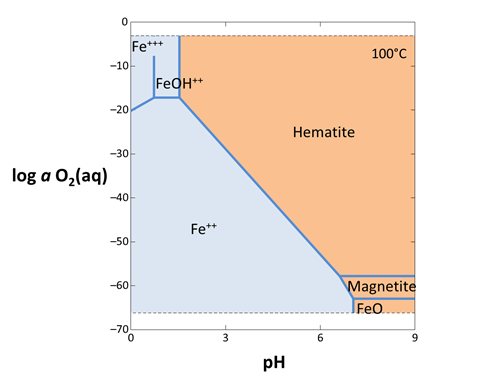
Act2 calculates stability diagrams in activity and fugacity coordinates. To calculate a redox-pH diagram for iron, for example, start Act2 from the GWB dashboard
and move to the Basis pane:
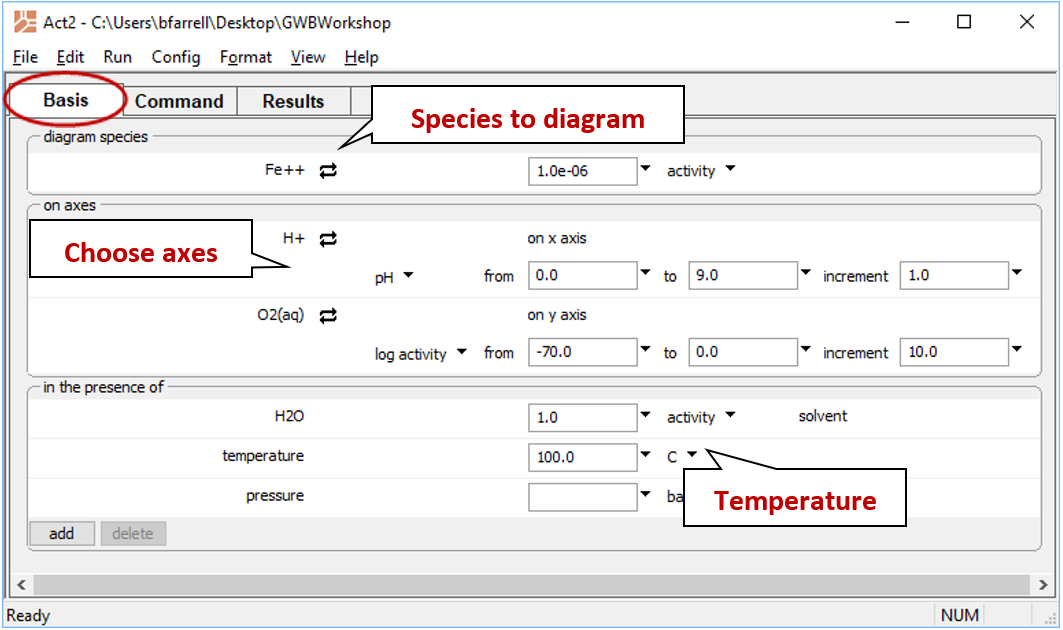
- Under “diagram species”, select “Fe++” and set activity to “10^-6”.
- Under “on axes”, select “H+” for x and set the unit to “pH”. Set the axis to range up to “9”.
- For y, choose “O2(aq)” set the axis to range from “−70.0” to “0”.
- Set “temperature” to 100°C.
The pane should look like
Move to the Plot pane to view your diagram.
The dashed lines that limit the top and bottom of the plot show the stability limits of water at 1 atmosphere pressure. The diagram shows various aqueous species, minerals, and gases that are predominant under the chosen conditions. Each is formed from some combination of species in our basis set: Fe++, H+, O2(aq), and H2O.
Each solid line on the diagram represents a reaction between species at equilibrium. You can click on a line to see its stoichiometry in a tooltip. In Rxn, you can additionally find the equation for the equilibrium line, as described in the Equilibrium lines section of the Online Academy.
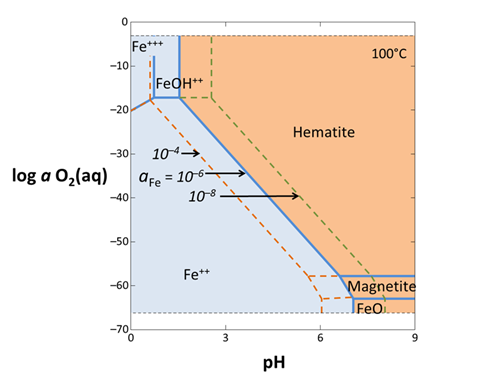
Return to the Basis pane and recalculate the diagram assuming Fe2+ activities of 10−4 and 10−8. (Note that CTRL+G in the GWB apps is a shortcut for Run → Go.) Comparing the three plots, do the positions of the reaction lines between aqueous species change? The reaction lines between minerals?
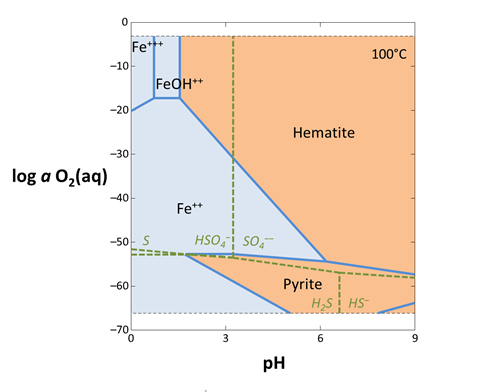
You can add complexing species to the diagram on the Basis pane, under “in the presence of”. Some complexing species react across the diagram coordinates. For example, SO42− reacts under changing Eh or pH to form HSO4−, HS−, H2S(aq), and so on. To correctly include SO42− as a complexing species, we need to calculate a mosaic diagram.
Revert the Fe2+ activity to “10^-6”. Under “in the presence of”, click on add and select “SO4--”. Set the activity to “0.001”, then click the pulldown next to “activity” and select “speciates over x-y”
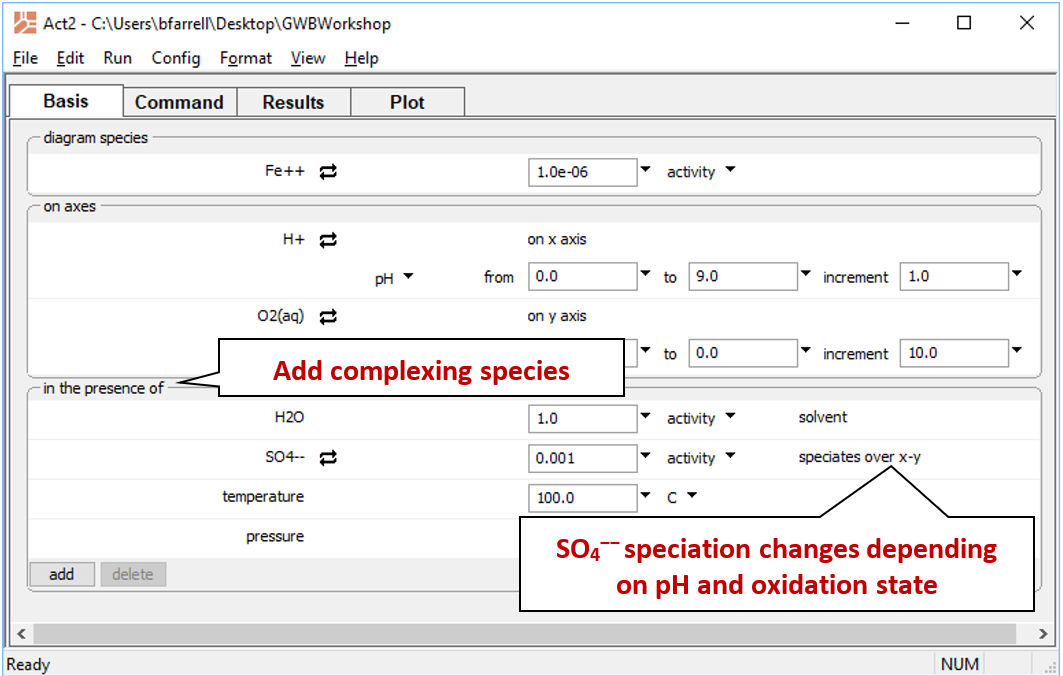
Return to the Plot pane to view your updated diagram.
How did your diagram change? How does the plot look if you don't check “speciate over x-y”?
Task 2: Stability diagrams
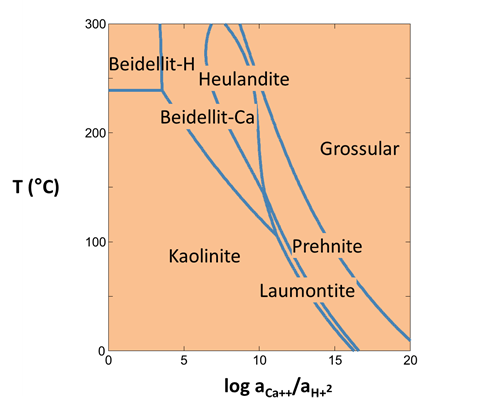
You can diagram stability vs. temperature using Tact. To diagram the thermal stability of calcic aluminosilicates in the presence of quartz, start Tact
and move to the Basis pane.
- Under “diagram species” set “Al+++” and then
 Mineral… → “Kaolinite”.
Mineral… → “Kaolinite”. -
On the x axis, select “Ca++”, then click on
 Ratio… Set “Ca++” for the numerator, and “H+” squared in the denominator with an exponent of "2".
Ratio… Set “Ca++” for the numerator, and “H+” squared in the denominator with an exponent of "2".
- Set the axis range from “0” to “20”.
- Under “in the presence of”, add “SiO2(aq)” and swap
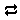 Mineral… → “Quartz”.
Mineral… → “Quartz”.
The pane should look like
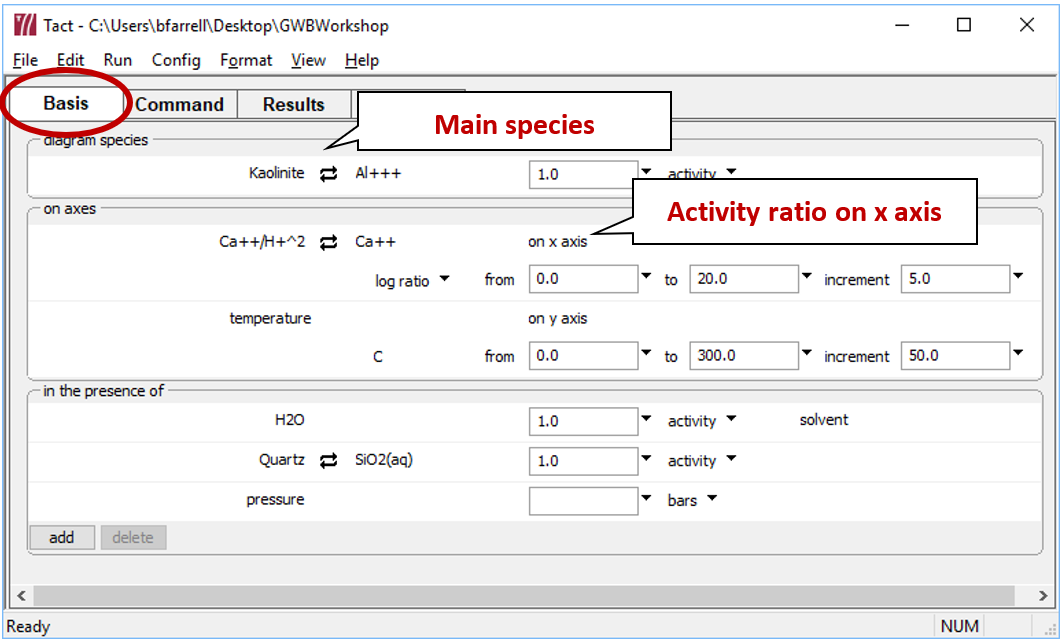
Move to the Plot pane to view your diagram. Under what conditions are clay minerals stable? Zeolites?
Task 3: Solubility diagrams
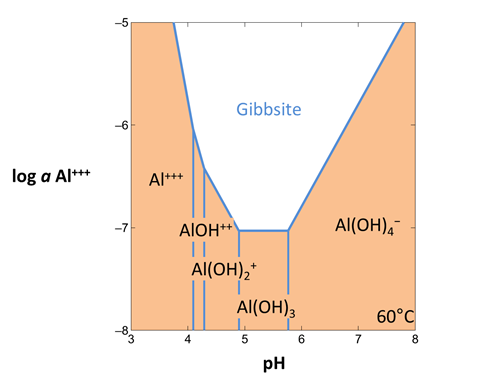
To diagram the solubility of a mineral, open Act2 and set the main species being diagrammed as an axis species. For example, to represent solubility at 60°C in the alumina-water system,
- Under “diagram species”, select “Al+++”.
- Under “on axes”, set “H+” and units of “pH” for x. Set a range from “3” to “8”.
- For the y axis, select “Al+++” and a range from “−8” to “−5”.
- Set “temperature” to 60°C.
The pane should look like
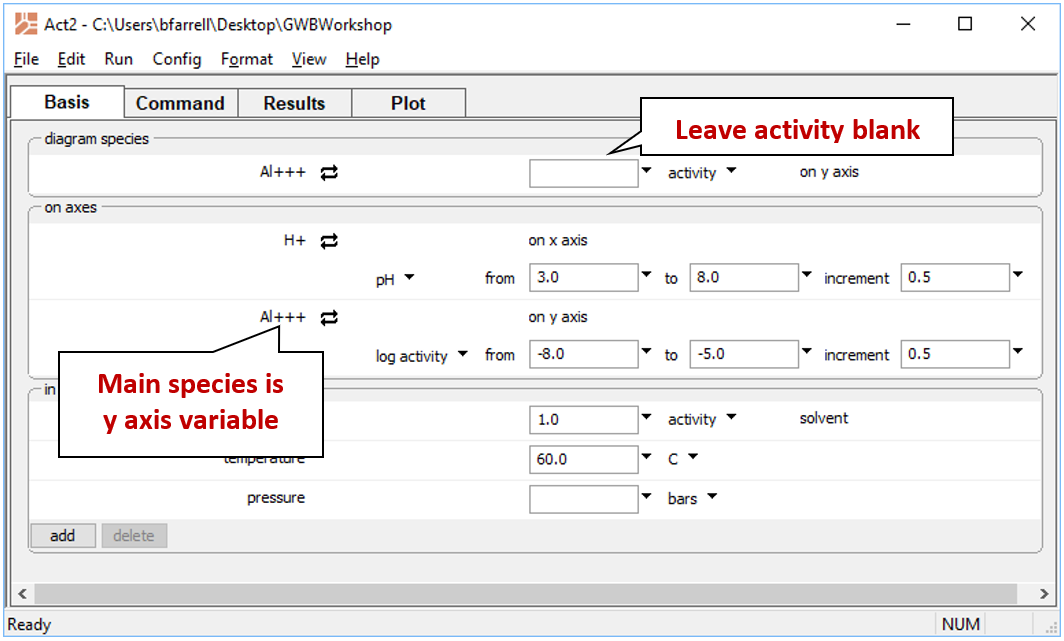
Move to the Plot pane to view your diagram. What kinds of reactions are represented by vertical lines? Horizontal lines?
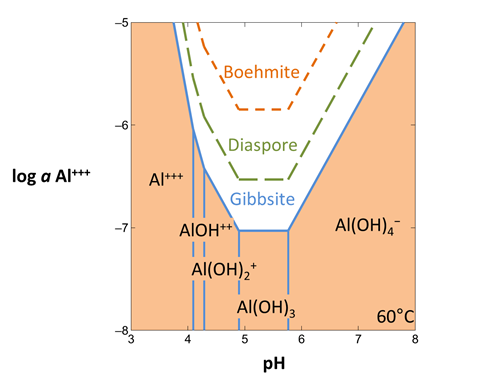
To diagram the solubility of a less stable alumina mineral, suppress the more stable form. In this way, you prevent Act2 from considering a particular mineral or species while calculating the diagram.
From the Config → Suppress… dialog
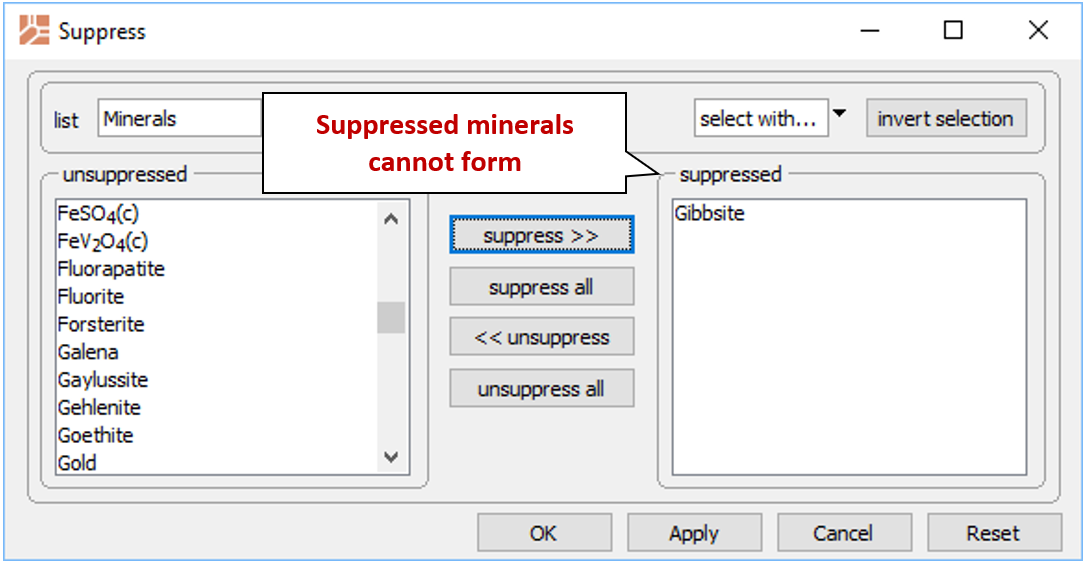
select “Gibbsite” and then suppress >> to prevent it from forming. Take a look at the resulting plot. Suppress “Diaspore” and calculate the diagram once again.
Authors
Craig M. Bethke and Brian Farrell. © Copyright 2016–2026 Aqueous Solutions LLC. This lesson may be reproduced and modified freely to support any licensed use of The Geochemist's Workbench® software, provided that any derived materials acknowledge original authorship.
References
Bethke, C.M., B. Farrell, and M. Sharifi, 2026, The Geochemist's Workbench®, Release 18: GWB Essentials Guide. Aqueous Solutions LLC, Champaign, IL, 232 pp.
Comfortable with stability diagrams?
Move on to the next topic, Reactions and Equilibrium Lines, or return to the GWB Online Academy home.