Mobile colloids
Mobile Colloids is part of a free web series, GWB Online Academy, by Aqueous Solutions LLC.
What you need:
- GWB Professional recommended
-
Input file:
 Colloid.x1t
Colloid.x1t
Download this unit to use in your courses:
- Lesson plan (.pdf)
- PowerPoint slides (.pptx)
Click on a file or right-click and select “Save link as…” to download.
Introduction
Colloids are small particles entrained in flowing groundwater. Because of their high surface area, many are highly reactive. Examples include clay minerals, silica-rich particles, iron oxides, humic macromolecules, bacteria, and viruses.
In The Geochemist's Workbench®, we define colloids as mobile sorbing surfaces. A mobile colloid consists of the mineral or minerals associated with a complexing surface (two-layer, three-layer, and CD-MUSIC models), as well as the ions it has complexed.
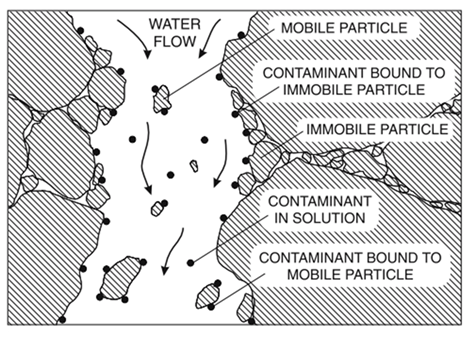
The figure below illustrates how transport of a contaminant is highly dependent on the mobility of the sorbent
All complexing surfaces have a mobility, the fraction of a surface that can move by advection and dispersion:
- 0, the default, sets the surface to be stationary
- 1, the surface moves freely
- Can be intermediate between 0 and 1
Task 1: Mobile colloids
Let's construct a model of how a ferric colloid migrating with groundwater displaces Pb2+ from contaminated aquifer sediments.
To start, double-click on file “Colloid.x1t”. The configuration is much like the earlier example (“3metals.x1t”), except the aquifer is already contaminated with Pb2+. A colloid migrates into the aquifer and strips the heavy metal from the aquifer sediments as it travels down-gradient.
Moving to the Initial pane
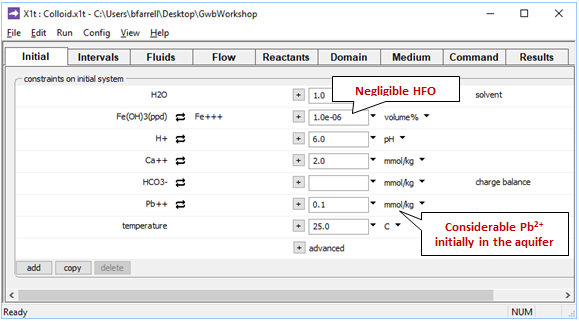
we see that the aquifer contains a negligible amount of the ferric precipitate Fe(OH)3, a Ca-HCO3 water, and a considerable amount of inorganic lead distributed between solution and sorbate.
On the Fluids pane
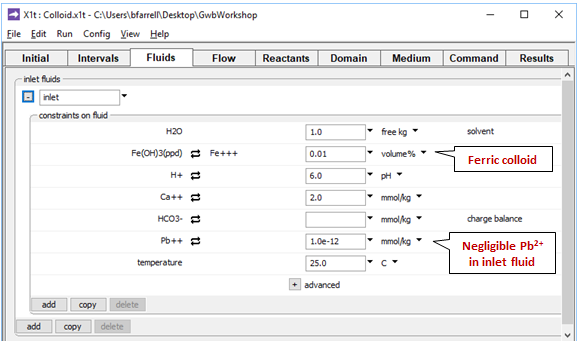
the fluid passing into the domain is the same as the initial fluid, except it contains more abundant ferric precipitate, but almost no Pb2+.
Opening Config → Sorbing Surfaces…, we see we've read in the “Kd.sdat” and “FeOH.sdat” datasets
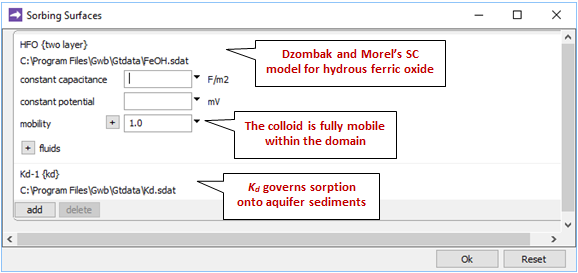
The former supplies a distribution coefficient, Kd, governing sorption of Pb2+ onto the aquifer sediments, which are immobile. The latter contains Dzombak and Morel's (1990) compilation of surface complexation reactions for hydrous ferric oxide.
We'll set the mobility of the ferric colloid everywhere in the domain to 1 in order to make the Fe(OH)3 precipitate and its sorbate into a mobile colloid. Its mobility in the inlet fluid is by default set equal to that in the first interior node in the domain, which in this case is 1.
On the Config → Redox Couples… dialog
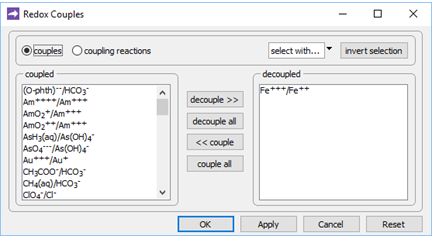
we see the redox coupling between ferric and ferrous iron has been disabled. This setting explains why we've been able to include Fe3+ species and minerals in the simulation, without setting basis entries representing Fe2+ or redox state.
Going to Config → Options…
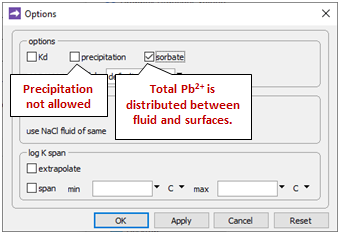
the “precipitation” option has been deselected. This setting prevents stable but slow-forming ferric minerals like goethite (FeOOH) from appearing in the simulation, at the expense of the Fe(OH)3 precipitate.
We also see that the “sorbate include” option has been selected. As a result, the total Pb2+ concentrations we specify on the Initial and Fluids panes are in each case distributed between the fluid and the sorbate.
On the Config → Suppress… dialog
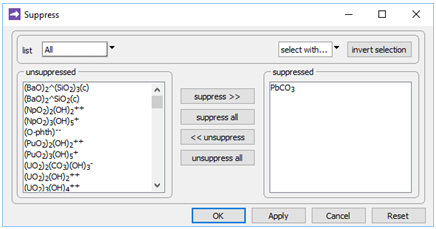
we've suppressed the PbCO3 ion pair, the stability of which in the database is almost certainly erroneous.
Finally, on Config → Output…, the suffix “_colloid” will be applied to datasets produced by the run
Trace the simulation by selecting Run → Go.
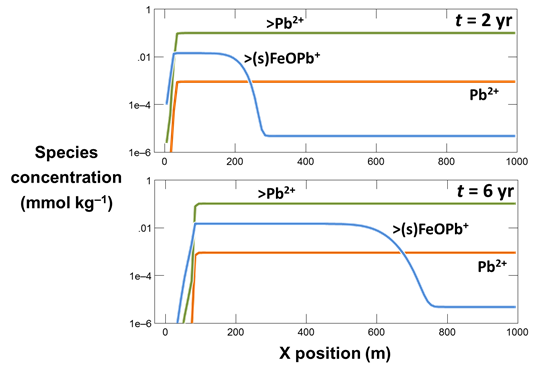
Use Xtplot to make a graph showing the mass of the sorbing mineral Fe(OH)3 throughout the domain. Hint: double-click on the x-axis label and for “Display” choose “X position”
On the Y Axis pane choose “Minerals” and then select “Fe(OH)3(ppd)”
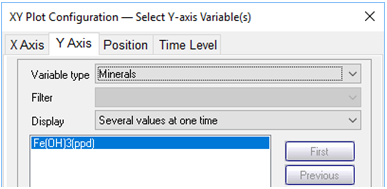
What transport processes appear to affect the colloid (the mineral and its sorbate)?
Now, plot the concentrations of the various Pb2+ species along the aquifer. On the Y Axis pane choose “Species concentration” for “Variable type” and select “Pb++” for “Filter”. Then select “Pb++”, “>Pb++”, and “>(s)FeOPb+”
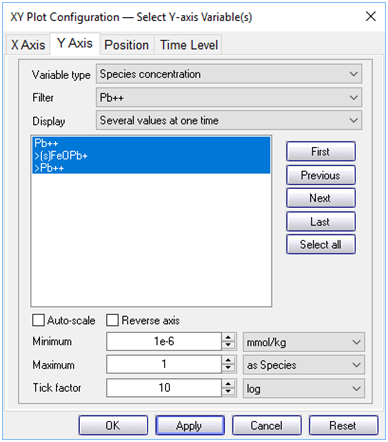
What effect does the colloid have on the transport of Pb2+?
Authors
Craig M. Bethke and Brian Farrell. © Copyright 2016–2026 Aqueous Solutions LLC. This lesson may be reproduced and modified freely to support any licensed use of The Geochemist's Workbench® software, provided that any derived materials acknowledge original authorship.
References
Bethke, C.M., B. Farrell, and M. Sharifi, 2026, The Geochemist's Workbench®, Release 18: GWB Reactive Transport Modeling Guide. Aqueous Solutions LLC, Champaign, IL, 191 pp.
Grolimund, D., K. Barmettler, and M. Borkovec, 2007, Colloid Facilitated Transport in Natural Porous Media: Fundamental Phenomena and Modelling. In: Frimmel, F., F. Kammer, & H. Flemming, Eds. Colloidal transport in porous media. Springer-Verlag, 291 pp.
Comfortable with mobile colloids?
Move on to the next topic, Biodegradation, or return to the GWB Online Academy home.