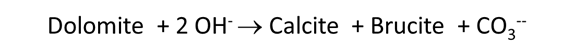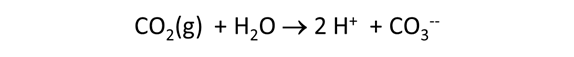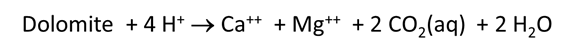Waste injection wells
Waste Injection Wells is part of a free web series, GWB Online Academy, by Aqueous Solutions LLC.
What you need:
- GWB Standard recommended
-
Input files:
 dolomite.rea,
dolomite.rea,  blowout.rea
blowout.rea
Download this unit to use in your courses:
- Lesson plan (.pdf)
- PowerPoint slides (.pptx)
Click on a file or right-click and select “Save link as…” to download.
Introduction
Various industries around the world that generate large volumes of liquid byproducts dispose of their waste by injecting it into the subsurface. Because of environmental concerns and the potential difficulties posed by losing permeability and formation caving, well operators seek to avoid deterioration of the formation accepting the wastes or the formation's confining layers. Wastes are commonly far from chemical equilibrium with the minerals in the formation when they are injected and, as such, can be expected to react extensively with it (Boulding, 1990).
Task 1: Formation damage
First, we consider injection of caustic waters into Devonian dolomites at a depth of about 2600 feet. About 6 million gallons of waste were injected monthly into two wells.
At the time of drilling, analysis of formation samples indicated that the injection zone was composed of nearly pure dolomite, CaMg(CO3)2. The carbonate formation was thought to be safe for accepting an alkaline wastewater because carbonates are considered stable at high pH.
With time, however, well operators encountered a number of problems, including formation caving and loss of porosity and permeability in zones that had been accepting fluid.
Double-click on file “dolomite.rea” and when React opens, look at the Basis pane
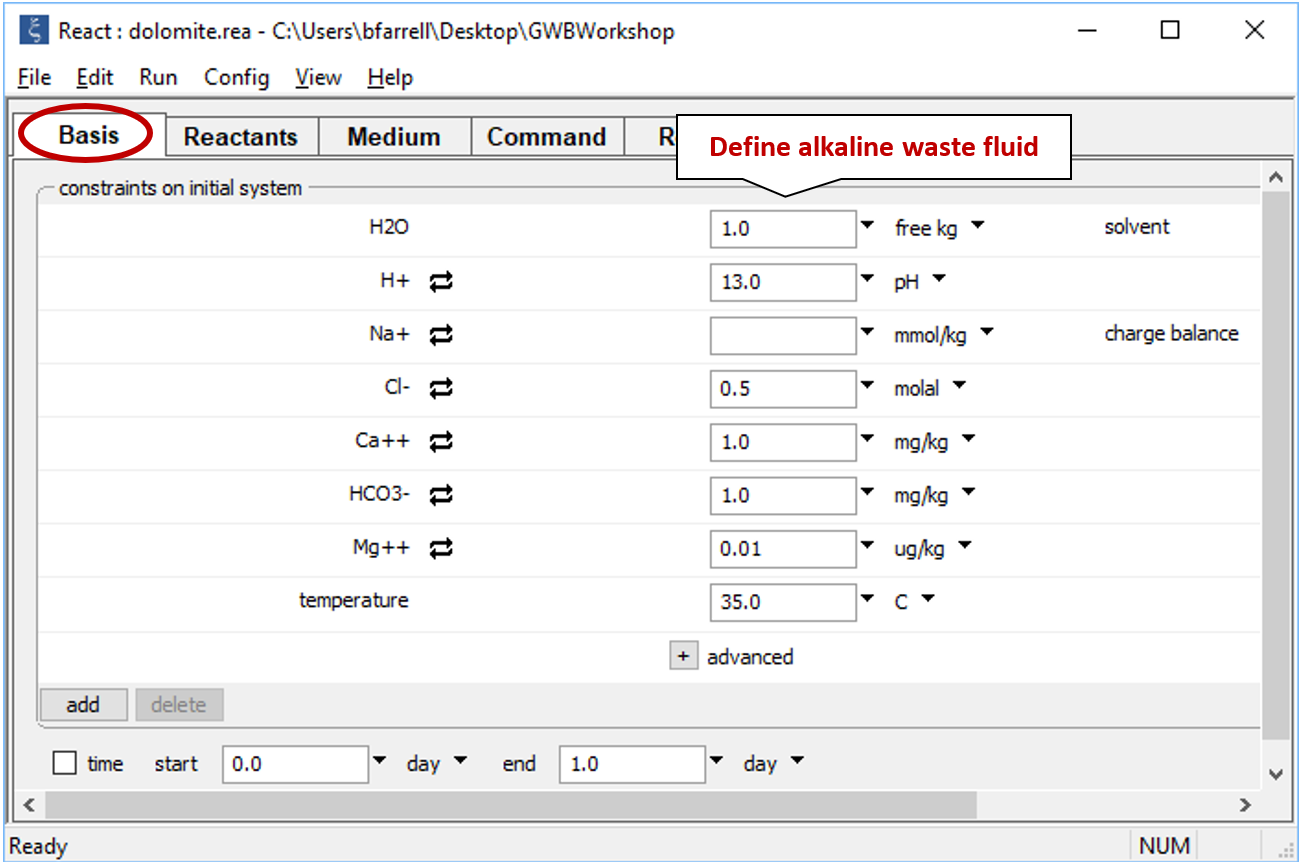
which contains the composition of the alkaline waste.
Move to the Reactants pane
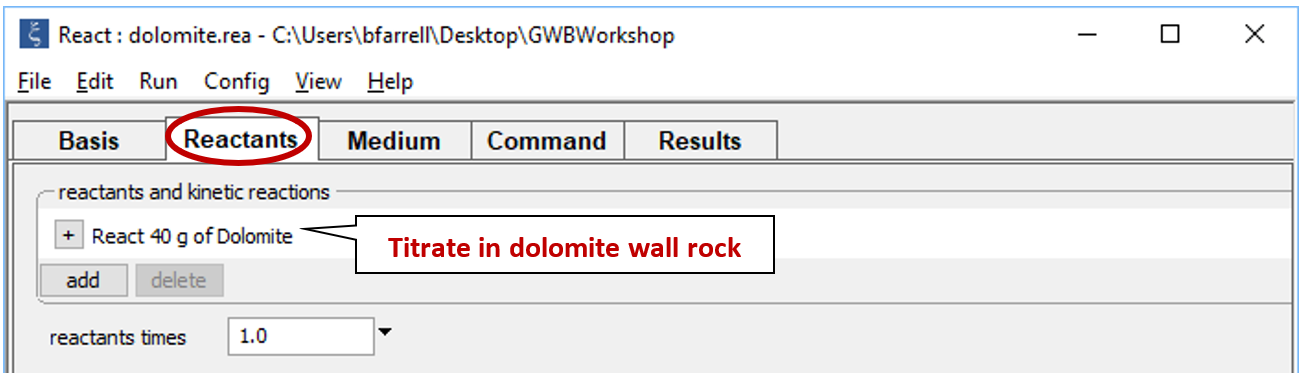
The input sets up the irreversible reaction of dolomite into the NaOH-NaCl waste.
On Config → Output... set a suffix “_dolomite”
Click OK, then on the main window select Run → Go.
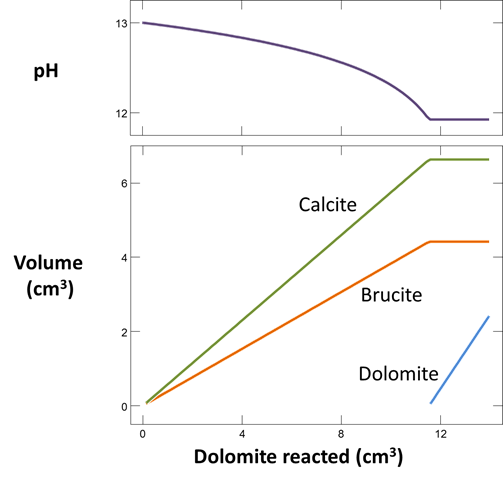
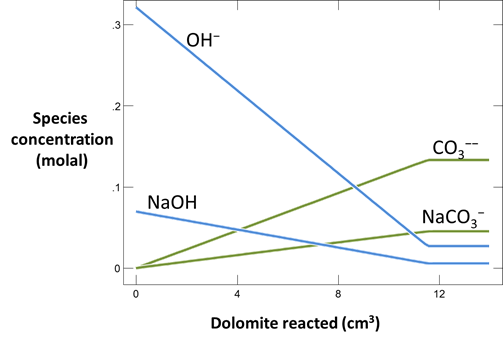
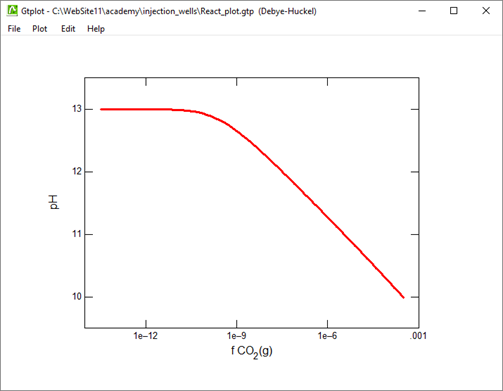
Plot the minerals that, according to the calculation, form as dolomite dissolves. Also plot how dolomite dissolution affects the solution pH and species distribution. Write the overall reaction predicted.
In June, 1987, a number of sidewall cores were taken from the formation (Mehnert et al., 1990). XRD analysis showed that significant amounts of calcite (CaCO3) and brucite (Mg(OH)2), as well as some amorphous material, had formed from the original dolomite. In some cases, the dolomite was completely consumed and the rock found to be composed entirely of a mixture of calcite and brucite. Do these observations agree with your calculation?
Suppose that before it was injected, the waste was left in a lagoon long enough to equilibrate with CO2 in the atmosphere. Would the waste remain corrosive to dolomite?
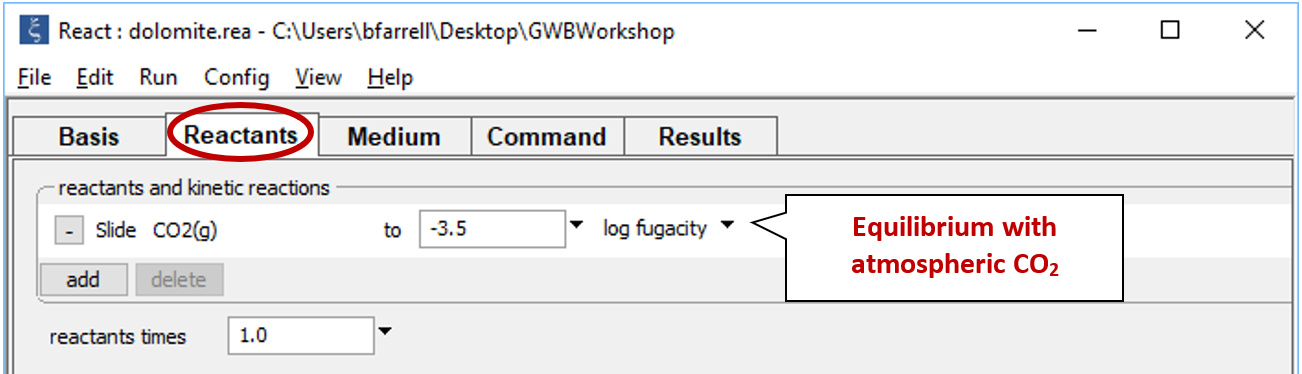
Starting with the previous run, we'll remove the simple reactant dolomite from the Reactants pane, and instead specify a reaction path in which CO2 fugacity is increased to its value in the atmosphere, as shown below.
Select the “React 40 g of Dolomite” entry and click  , then go to
, then go to  → sliding → gas → “CO2(g)”. Expand the entry by clicking on the
→ sliding → gas → “CO2(g)”. Expand the entry by clicking on the  , change the unit to “log fugacity”, and set a value of “−3.5”, the partial pressure of CO2 in the atmosphere
, change the unit to “log fugacity”, and set a value of “−3.5”, the partial pressure of CO2 in the atmosphere
Select Run → Go to begin the sliding fugacity path.
What is the effect of equilibrating the waste with CO2 in the atmosphere?
Now that the fluid has been equilibrated with atmospheric CO2, we'll see how it reacts with the dolomite formation. First, take the results of the path we just traced and use it as the starting point of a new run: Run → Pickup → System → Fluid. Move to the Basis pane to confirm the new starting fluid.
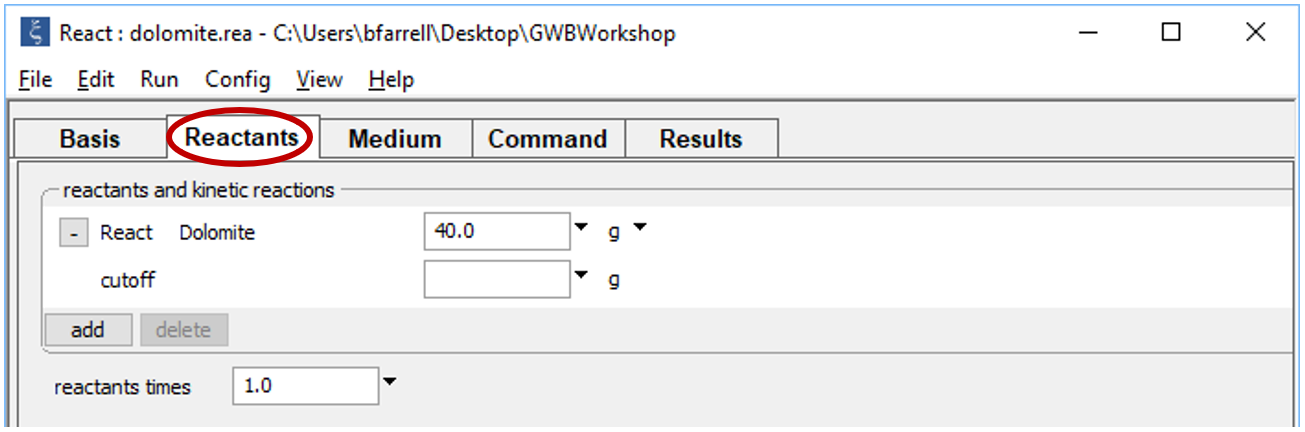
Move to the Reactants pane and once again set 40 grams of dolomite as a simple reactant, to be titrated into the equilibrated waste fluid. Choose  → Simple → Mineral… → “Dolomite”. Expand the field, set units to “g”, and enter a value of “40”. Leave the “cutoff” entry empty
→ Simple → Mineral… → “Dolomite”. Expand the field, set units to “g”, and enter a value of “40”. Leave the “cutoff” entry empty
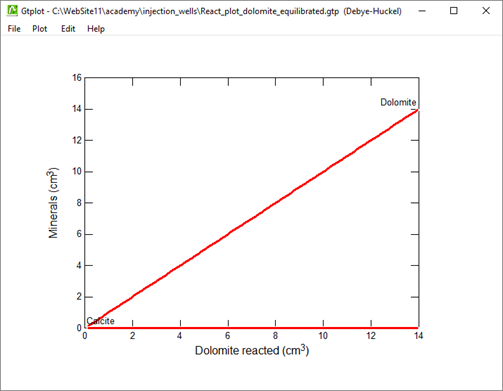
Set a suffix “_dolomite_equilibrated” on Config → Output... then click Run → Go to rerun the simulation.
Replot the results. How much dolomite dissolves in this case?
Task 2: Gas blowouts
Hydrochloric acid waste at Cabot Corporation near Tuscola, Illinois is injected into the Cambrian Eminence and Potosi formations, which are largely dolomites, at about 5,000 feet (Brower et al., 1989). In the 1980s, the well suffered a series of gas blowouts.
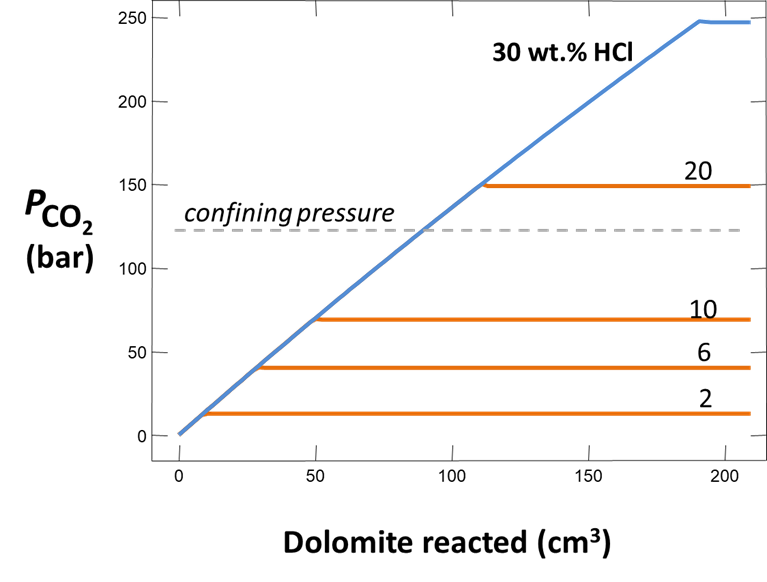
Blowouts appear to have occurred at times when especially acidic wastes were being injected. The acid apparently reacted with carbonate in the formations to produce a CO2 gas cap at high pressure. In some cases, the injected waste was more than 31 wt.% HCl. As a temporary measure, the plants are now limited by the Illinois Environmental Protection Agency (IEPA) to injecting wastes containing no more than 6 wt.% HCl.
Use React to investigate how HCl wastes would be expected to interact with dolomite in injection zones. The following table converts wt.% HCl to molality:
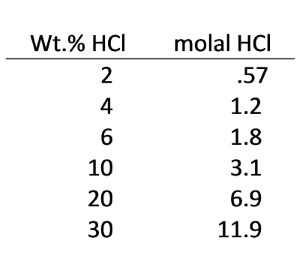
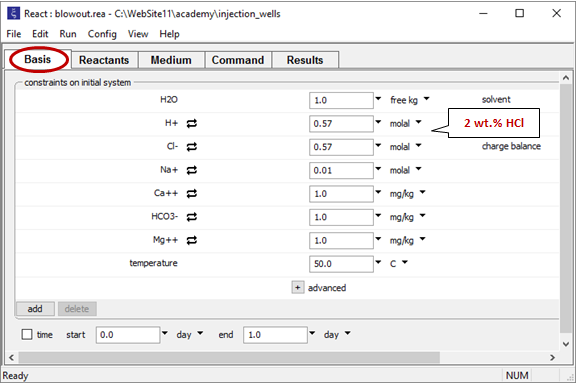
Double-click on file “blowout.rea” and move to the Basis pane
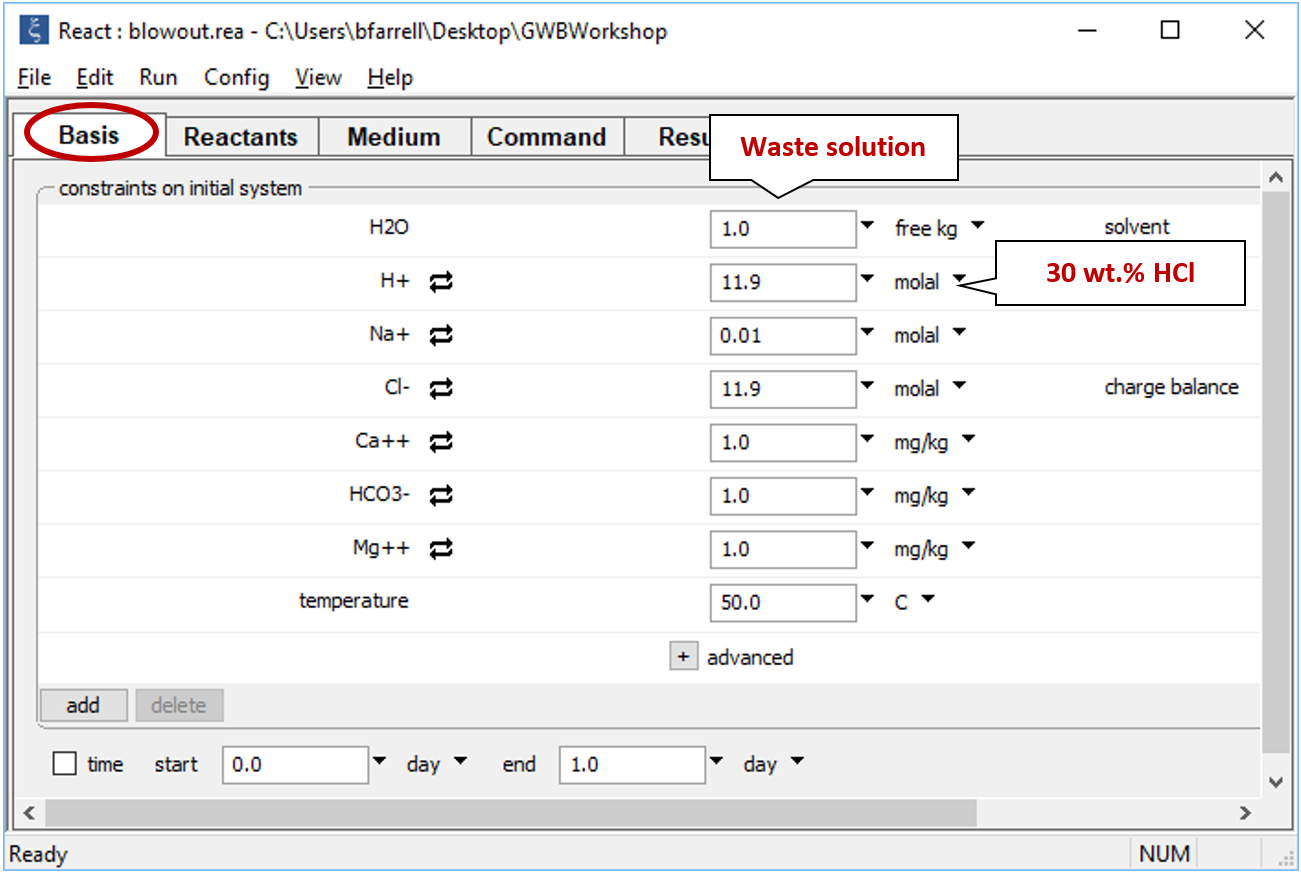
The pane describes the acidic waste fluid.
Move to the Reactants pane
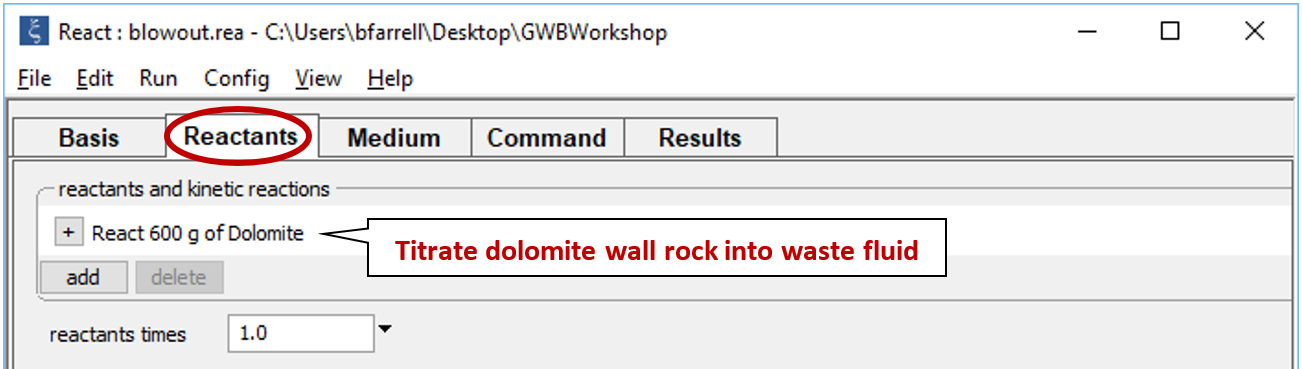
Here we've defined a reaction path in which we'll titrate 600 g of dolomite into the waste fluid.
Set a suffix “_blowout”
and select Run → Go.
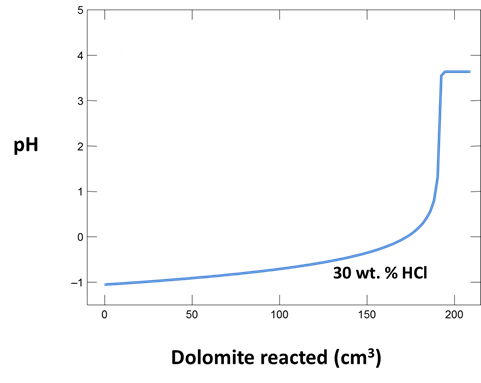
What is the initial pH of the injected solution? Analyses of injected fluids report pH as <1 because the solutions are too acidic for available pH electrodes. Is this in agreement with your calculation?
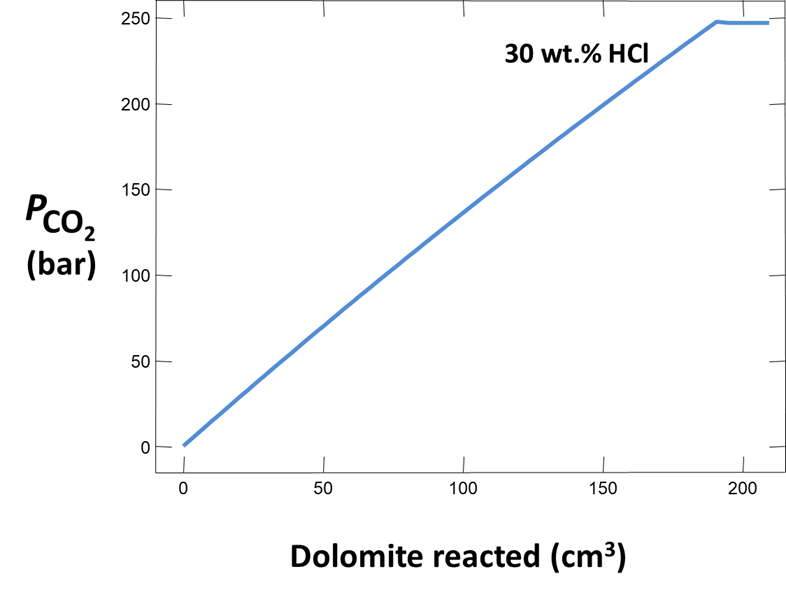
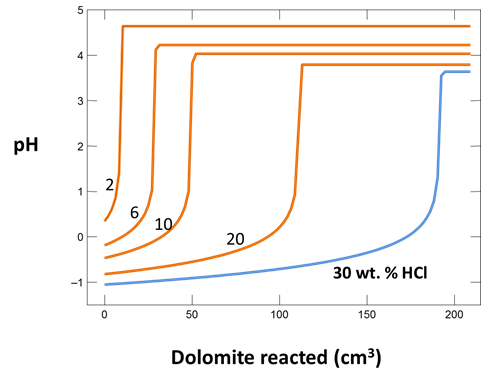
Plot how pH and CO2 partial pressure vary as dolomite reacts with the fluid. How much dolomite can be dissolved in a kg of a solution of this acidity? What is the overall reaction?
Does the CO2 partial pressure exceed the fluid pressure of about 120 atm at these depths? Could you have predicted the risk of blowing out a well by injecting such an acidic solution?
Authors
Craig M. Bethke and Brian Farrell. © Copyright 2016–2026 Aqueous Solutions LLC. This lesson may be reproduced and modified freely to support any licensed use of The Geochemist's Workbench® software, provided that any derived materials acknowledge original authorship.
References
Bethke, C.M., 2022, Geochemical and Biogeochemical Reaction Modeling, 3rd ed. Cambridge University Press, New York, 520 pp.
Bethke, C.M., B. Farrell, and M. Sharifi, 2026 The Geochemist's Workbench®, Release 18: GWB Reaction Modeling Guide. Aqueous Solutions LLC, Champaign, IL, 219 pp.
Boulding, J.R., 1990, Assessing the geochemical fate of deep-well-injected hazardous waste. US Environmental Protection Agency Report EPA/625/689/o25a.
Brower, R.D., A.P. Visocky, I.G. Krapac, B.R. Hensel, G.R. Peyton, J.S. Nealon and M. Guthrie, 1989, Evaluation of underground injection of industrial waste in Illinois. Illinois Scientific Surveys Joint Report 2.
Mehnert, E., C.R. Gendron and R.D. Brower, 1990, Investigation of the hydraulic effects of deep-well injection of industrial wastes. Illinois State Geological Survey Environmental Geology 135, 100 p.
Comfortable with injection wells?
Move on to the next topic, Hydrothermal Fluids, or return to the GWB Online Academy home.